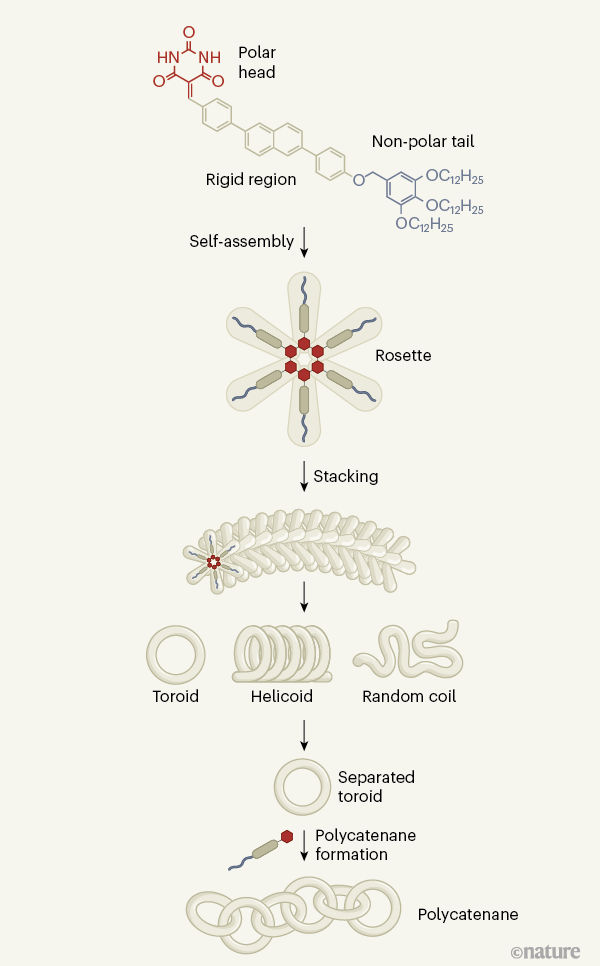
Sophisticated molecular architectures are typically built by connecting a range of setting up blocks jointly in a stepwise manner. But from time to time, advanced constructions emerge from the self-assembly of a solitary constituent. Producing in Character, Datta et al.1 display how polycatenanes — chains designed of interlocked nanometre-scale rings — can be fashioned by the remarkable self-assembly of a straightforward molecular developing block.
Catenanes are molecules in which two or far more molecular rings are entangled like the back links of a chain2 without a doubt, their name derives from catena, the Latin term for chain. The rings are not connected by a covalent bond, but rather variety a diverse sort of linkage referred to as a mechanical bond, in which the related rings can shift freely close to each other. This dynamic property tends to make them useful as elements of artificial molecular equipment3. A lot of catenanes reported so significantly consist of only two rings. The design of molecular chains manufactured of numerous rings is a significant obstacle for synthesis, and has been reached only in the past handful of yrs, for smaller molecular rings (with a radius of around 1 nanometre)4.
The building of greater techniques is minimal by the effectiveness of the catenation phase, in which a preassembled toroid precursor kinds a ring that interlinks through a further toroid furthermore, a large quantity of covalent bonds will have to be formed in the preassembled structure. Artificial routes that entail non-covalent assembly techniques are for that reason desired5. In supramolecular polymerization, for instance6, basic molecular setting up blocks self-assemble in a single action by means of non-covalent interactions to form large-scale constructions of diversified geometries. Regretably, the get in sizing of the assemblies that are designed in this way generally will come at a rate: chemists have fewer control of the closing construct’s architecture, in comparison with a multistep covalent approach.
Datta and co-workers have put together features of covalent and non-covalent strategies to form their elaborate polycatenane constructions. The authors commenced with a monomer composed of a polar head and a non-polar tail, divided by a rigid section consisting of benzene rings (Fig. 1). Six of these monomers can self-assemble in an suitable solvent to variety a star-formed ‘rosette’. The polar heads kind a hexagonal core that is held together by hydrogen bonds — in a lot the exact way that DNA helices are held collectively by hydrogen bonds in nucleotide base pairs — and the rigid sections stage outwards from the core like arms.
At the time shaped, the rosettes self-assemble by stacking on leading of just about every other — a procedure pushed by the development of interactions (acknowledged as π–π interactions) among the rigid locations of neighbouring rosettes. Because every single rosette additional to the stack is marginally offset from its predecessor, the ensuing assembly grows with an intrinsic curvature that produces various geometries: random coils, helicoids and toroids7. The form of geometry that varieties depends on the amount of cooling of the initial monomer alternative. Sluggish cooling (about 1 kelvin per minute) favours the formation of helical fibres more quickly cooling (about 10 K min–1) generates random coils and abrupt cooling adds toroids into the blend.
Datta and colleagues recognized that quick cooling also made traces of catenanes consisting of two interlocking toroids. This suggested that unique toroids could act as secondary sites from which a different ring could mature, thereby forming the catenated dimers. The authors took benefit of this fortuitous process to devise a protocol for producing large, self-assembled polycatenanes by employing a answer of toroids as ‘seeds’ for catenation.
The authors rapidly cooled a alternative of the monomer in a solvent mixture that was picked out to facilitate toroid formation, and thus created a solution in which somewhere around fifty percent of the monomer molecules ended up included into toroids the remaining monomers self-assembled into randomly coiled linear constructions. For the reason that the toroids are additional stable to warmth than are their linear counterparts, the authors could selectively disassemble the coils back again into monomers by heating the solution. Subsequent slow cooling promoted the development of prolonged, helical supramolecular assemblies from the monomers, leaving the toroids intact. Datta et al. then filtered the combination to eliminate the prolonged helical buildings, therefore manufacturing a remedy that predominantly contained toroids.
Finally, the authors developed polycatenanes by introducing monomers to the remedy of toroids, which seeded the development of new catenated rings, as had been hoped. The non-polar tails had been at first included into the monomers to strengthen monomer solubility, but Datta and colleagues identified that they also have a essential function in the seeding course of action: unfavourable interactions amongst the tails and the solvent will make it much more most likely that rosette self-assembly will initiate on the area of current toroids.
Atomic drive microscopy revealed that polycatenanes of various measurements type in the reactions, and that the toroids have a radius of 12.5 nm. The authors observed that addition of monomers in small portions favours the initiation of self-assembly processes that direct to catenation and ended up so equipped to make linear and branched polycatenanes containing up to 22 rings. This is near to the quantity previously realized using covalent assembly (up to 26 rings in linear polycatenanes)4, and additional demonstrates the efficiency of Datta and colleagues’ technique for synthesizing sophisticated, non-covalent buildings.
The authors’ protocol also exhibits that a multistep technique, borrowed from the covalent-synthesis playbook, can be made use of to make huge and elaborate self-assembled architectures in a controllable way. This is an essential phase in the development of non-covalent synthesis, and it can be predicted that their protocol will inspire the area to deal with more-demanding targets5. It would be interesting to see, for illustration, whether the monomer can be adapted to attain catenated rings of different dimensions, or regardless of whether hetero-catenanes can be made, in which the seed toroid is made up of a distinct style of monomer from the one from which the catenated macrocycles are assembled.
It continues to be to be noticed how the mechanical and dynamic homes of the self-assembled polycatenanes examine with people of their smaller sized covalent counterparts. A key attractiveness of covalently assembled catenanes is that, if the relative motion and place of the rings can be managed, it opens up probable programs for molecular devices. The chance of reaching the exact same amount of management about large, self-assembled buildings would convey us a tiny closer to what nature achieves with mobile equipment.

Analyst. Amateur problem solver. Wannabe internet expert. Coffee geek. Tv guru. Award-winning communicator. Food nerd.